ABSTRACT
Recently, the survival benefit of immuno-oncologic (IO) agents for advanced hepatocellular carcinoma (HCC) has been proven in several randomized controlled trials. Especially, atezolizumab with bevacizumab (Ate+Beva), as a first-line therapy for advanced HCC, has shown outstanding efficacy in the IMBrave150 study. Fortunately in south Korea, the cost of Ate+Beva therapy can be covered by national medical insurance, therefore HCC patients can receive Ate+Beva therapy without trouble. However, almost all HCC patients have no choice but to stop the treatment after two years completion of Ate+Beva therapy because our national medical insurance only cover IO therapy for two years. Therefore clinicians have been restarting the systemic treatment after confirming the disease progression of HCC patients on resting period of systemic therapy. Here, we report a case that showed a partial response of lymph node metastasis by 3rd line regorafenib therapy for progression of LN metastasis after achieving nearly complete response by two-year completion of 1st line Ate+Beva therapy in advanced HCC patient.
-
KEYWORDS: Hepatocellular carcinoma; Atezolizumab; Bevacizumab; Regorafenib; Radiotherapy
INTRODUCTION
Atezolizumab plus bevacizumab (Ate+Beva) proved better efficacy compared to sorafenib as a first line systemic therapy for advanced hepatocellular carcinoma (HCC) [
1]. However, there is no data about further treatment plan after 2 years completion of Ate+Beva therapy because in south Korea, almost all patients have no choice but to discontinue Ate+Beva therapy due to 2 years limitation of national insurance coverage. According to the Korean national medical insurance policy, HCC patients have to pay only 5% of the total cost of Ate+Beva under special disease exemption, however the extent of cost coverage is only during the two years, thus HCC patients must pay 100% cost of Ate+Beva with no national support beyond this period.
Here, we report a case that showed a partial response (PR) of lymph node (LN) metastasis by combination treatment of radiotherapy and third line regorafenib therapy for progression of LN metastasis after achieving nearly complete response (CR) of LN metastasis by two-year completion of first line Ate+Beva therapy in advanced HCC patient.
CASE
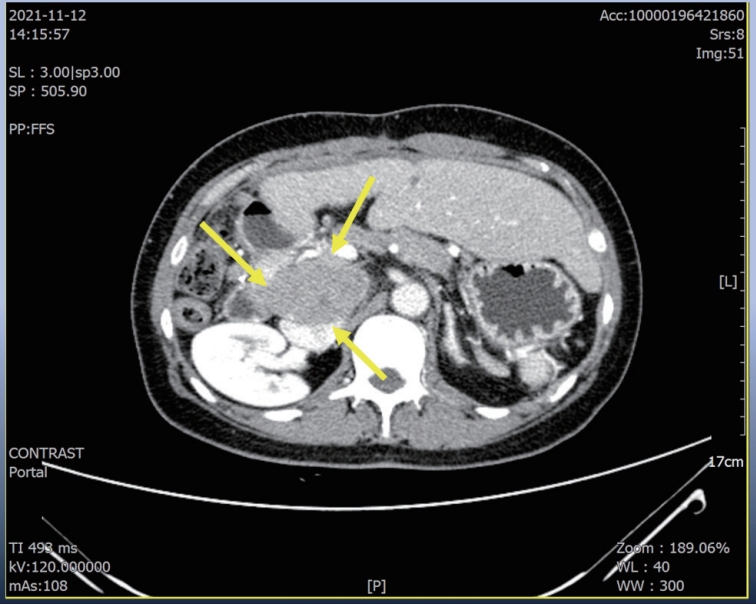
A 56-year-old woman with a multiple peritoneal and LN metastasis of HCC was admitted to our hospital from a nearby cancer center in November 2021. She already received right hemi-hepatectomy for initial HCC in 2018 and radiofrequency ablation for recurred tumor in 2019 and multiple times of excision for peritoneal & LN metastasis in 2021. However the metastasis was aggravated despite of previous several times of metastasectomy. Computed tomography (CT) revealed multiple LN & peritoneal metastasis (maximum diameter 6 cm) (
Fig. 1). The initial blood tests at the time of first visit in our hospital showed a white blood cell count of 6,010/mm
3, hemoglobin level of 13.5 g/dL, platelet count of 238,000/μL, aspartate aminotransferase concentration of 29 IU/L, alanine aminotransferase concentration of 20 IU/L, total protein level of 8.1 g/dL, total albumin level of 4.4 g/dL, prothrombin time of 76%, total bilirubin concentration of 0.5 mg/dL, and hepatitis C virus antibody was positive. She already received 12 weeks of sofosbuvir/ledipasvir (HARVONI
®; Gilead Science Inc., Foster City, CA, USA) and achieved sustained virologic response. The initial alpha-fetoprotein (AFP) level was above 15.1 ng/mL, and the protein level induced by the absence of vitamin K or antagonist-II (PIVKA-II) was 24 mAU/mL. Liver function was preserved with a Child-Turcotte-Pugh score of 5. The Eastern Cooperative Oncology Group (ECOG) performance status score was 0. Based on CT and LAB findings, the patient was identified as having advanced-stage HCC Barcelona Clinic Liver Cancer (BCLC) C stage. The patient was treated with Ate+Beva according to several guidelines. Atezolizumab (Tecentriq
® 1,200 mg per dose; Roche International LLC., Basel, Switzerland) and bevacizumab (Avastin
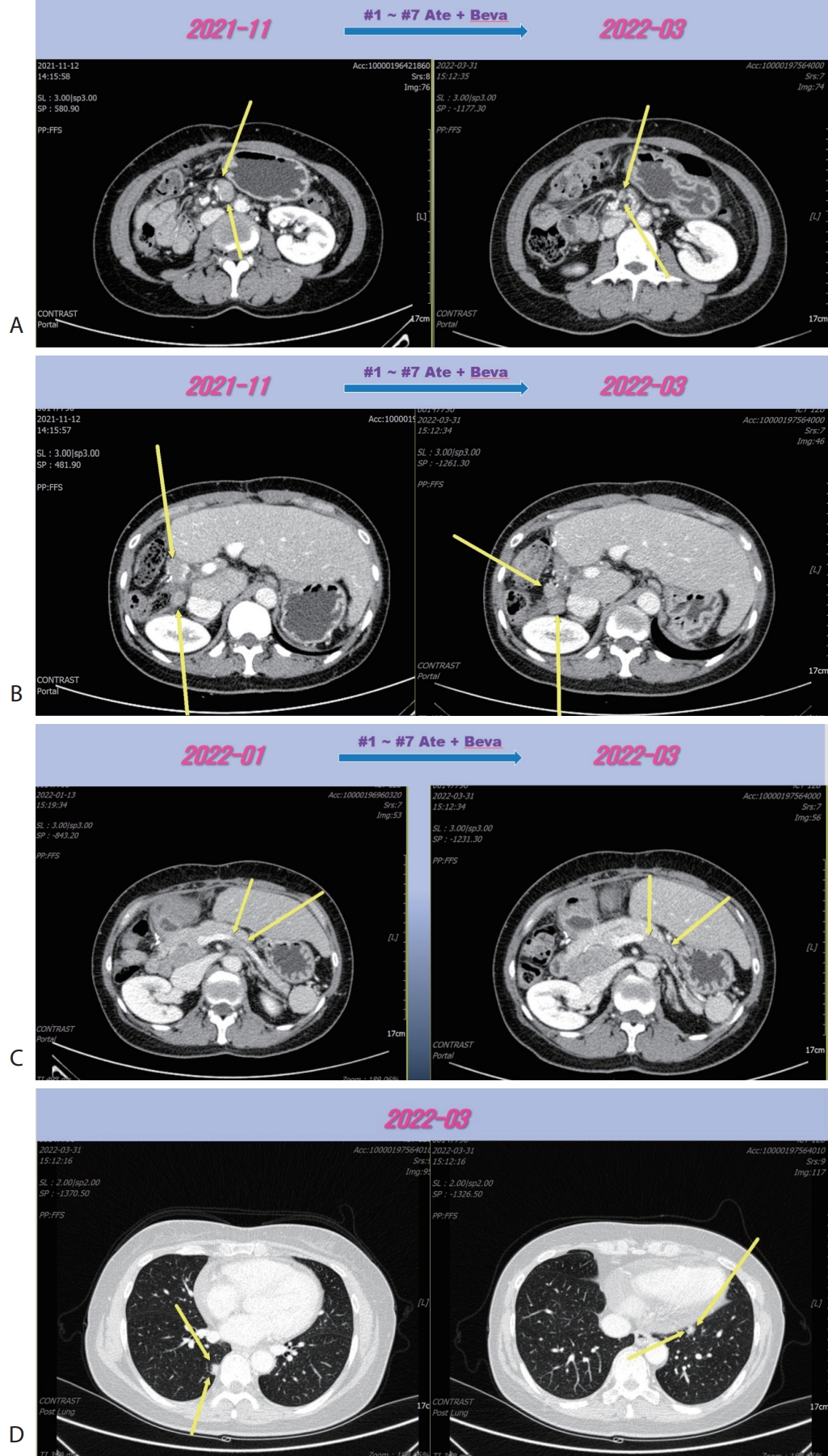
® 15 mg/kg per dose; Genentech, South San Francisco, CA, USA) were administered every 3 weeks on the same day. Following the seven cycles of Ate+Beva, liver CT scan revealed several peritoneal and LN metastasis were decreased compared to the prior CT scan (
Fig. 2A). However prerenal and peripancreatic LN metastasis were increased, moreover new onset lung metastasis was happened (
Fig. 2B–
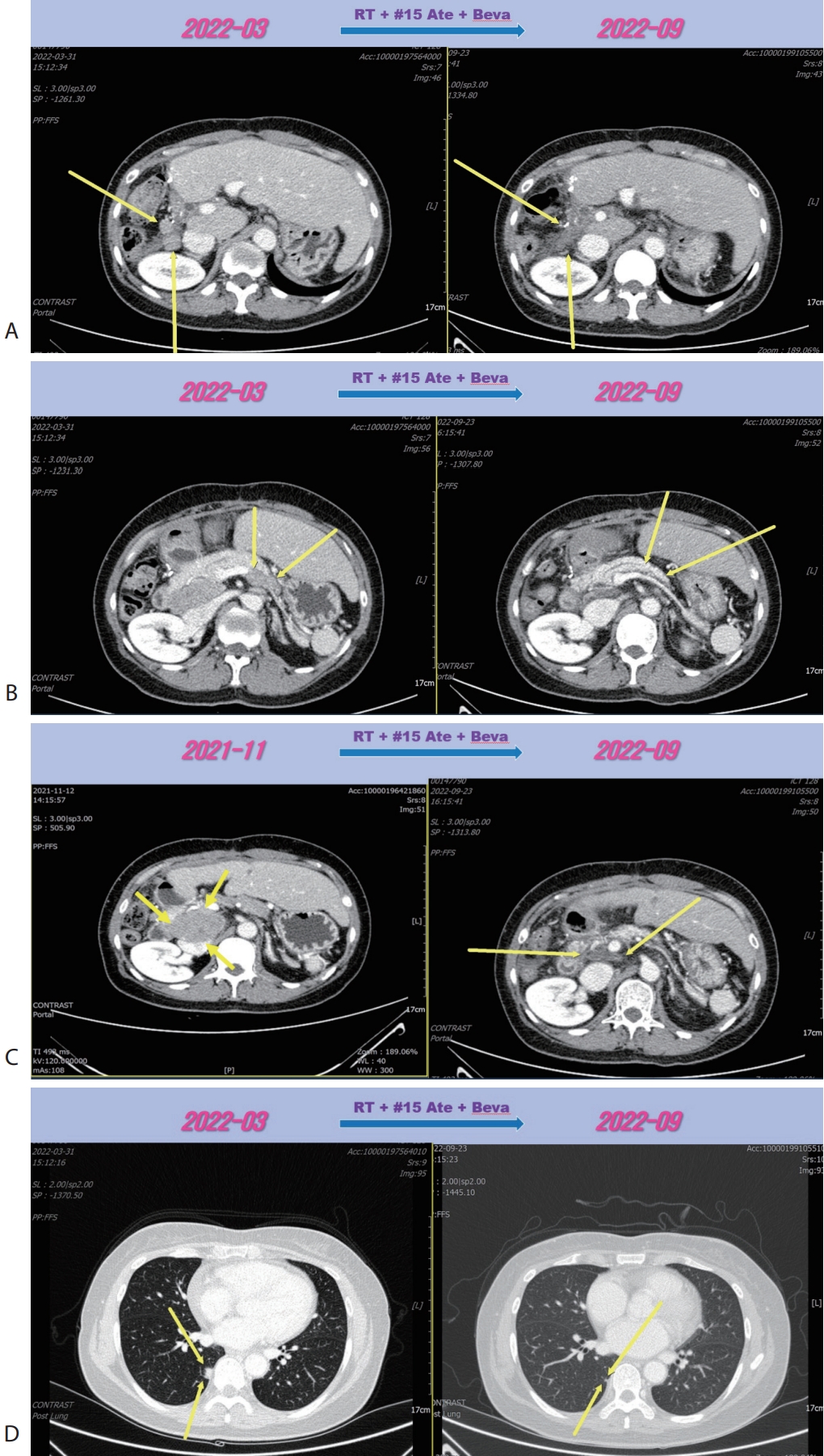
D). We concluded this mixed response as an oligo-progression and treated the patient with same systemic therapy (Ate+Beva) combining radiotherapy (RT) for prerenal LN metastasis (45 Gray/25 fraction). In follow-up CT scan that was conducted 6 months after commencing Ate+Beva plus RT, abdominal increasing LN metastasis previously was markedly shrinked (
Fig. 3A–
C) and lung metastasis was nearly disappeared (
Fig. 3D) and levels of AFP and PIVKA-II were normalized. We completed total thirty-two cycles of Ate+Beva for 2 years and we did not proceed further cycles of Ate+Beva because national medical insurance for Ate+Beva was terminated. At the time of stopping Ate+Beva, nearly CR state of tumor had been still maintained. However left inguinal & pelvic LN metastasis was recurred after 6 months from stopping Ate+Beva (
Fig. 4). Then the patient was treated with 2nd line sorafenib (Nexavar
®, 200 mg per dose; Bayer, Leverkusen, Germany) for 2 months, but LN metastasis was progressed (
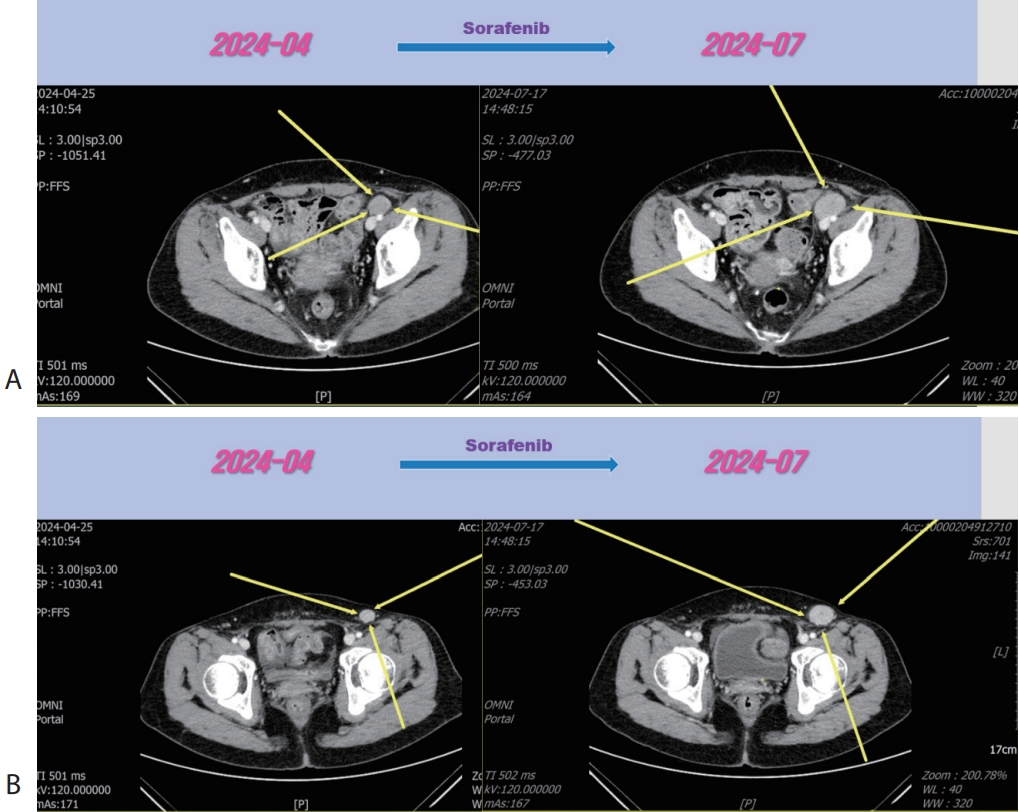
Fig. 4). Then the patient was treated with 3rd line regorafenib (Stivarga
®, 40 mg per dose; Bayer) for 3 months and LN metastasis was slightly increased (
Fig. 5), then we decided to combine RT for LN metastasis (40 Gray/16 fraction). Then the LN metastasis was markedly decreased (
Fig. 6). We are keeping on treating the patient with regorafenib therapy for over 12 months. We summarized treatment timeline of patient (
Fig. 7).
DISCUSSION
Most HCC is frequently advanced and incurable stage at the time of diagnosis [
2]. In one Korean nationwide population-based study, they reported the HCC patients received curative treatment was only 30.4% (surgical resection 21.3%, local ablation 8.4%, liver transplantation 0.7%) in 2022 [
3]. As a result, therapeutic options for the advanced stage of HCC are mainly intended to palliation therapy. Combination therapy with Ate+Beva was established as the initial treatment after demonstrating better outcomes in the IMBrave150 study compared to sorafenib. Ate+Beva had a 12-month OS rate of 67.2% (95% confidence interval [CI] 61.3–73.1), whereas sorafenib had an OS rate of 54.6% (95% CI 45.2–64.0). In the respective groups, the median progression free survival was 6.8 months (95% CI 5.7–8.3) and 4.3 months (95% CI 4.0–5.6) (hazard ratio for disease progression or death 0.59; 95% CI 0.47–0.76; p=0.001) [
1]. However, there is no data about further management after 2 years completion of Ate+Beva therapy because national medical insurance cover Ate+Beva therapy for only 2 years. Therefore in south Korea, if the patient cannot afford maintaining Ate+Beva therapy financially, clinician have no choice but to stop the treatment and wait for the disease progression.
Second or third line therapy after the disease progression with Ate+Beva has not been firmly concluded. Although the updated BCLC guidelines [
4] recommend carbozantinib as a salvage therapy after Ate+Beva treatment failure, the recommendation has not been clearly established with strong evidences based on the well-designed randomized controlled trials (RCTs). Practically in south Korea, there are only two regimens (sorafenib, lenvatinib) as the second line therapy after Ate+Beva. Some articles reported that PFS of lenvatinib was from 1.7 to 3.6 months longer than that of sorafenib. [
5-
7]. Further later line therapies are almost about treatment regimen in the setting of post-sorafenib failure. Several treatment regimens such as regorafenib [
8], cabozantinib [
9], ramucirumab [
10], showed 1.2–2.6 months longer PFS than that of placebo in each RCTs. Nivolumab+Ipilimumab showed recently meaningful clinical efficacy with the response rate of 32% and median OS of 22.8 months in phase II trial [
11]. On the basis of all these results, Korea Food and Drug Association approved all regimens we mentioned above as the next treatment after sorafenib failure.
In our case, we concluded the tumor response as the oligo-progression after initial seven cycles of Ate+Beva therapy because prerenal LN metastasis was increased and new-onset lung metastasis was detected although other LN and peritoneal metastasis are decreased. Although there is no clear definition of oligo-progression, the definition as followings is generally accepted; disease progression of 3–5 lesions in 1–3 organs after achieving at least stable disease of other sites for a minimum of 3 months on systemic therapy [
12,
13]. According to the concept of oligo-progression, if there are a few progressive sites in the context of overall stability of other tumor sites, treating these “oligo-progression sites” by local therapy, especially RT and maintaining the present systemic therapy might be helpful in select patients. RT can convert immune-suppressive tumor microenvironment to immune-sensitive because RT for tumor induce the release of tumor-associated antigens and activation of antigen-presenting cells, and stimulating active cytotoxic CD8+ T cell [
14]. In our case, combination therapy of RT with maintaining Ate+Beva resulted in nearly CR state of oligo-progressive sites (prerenal LN, lung) effectively.
In conclusion, we report a case that showed a PR of LN metastasis by 3rd line regorafenib therapy for progression of LN metastasis after achieving nearly CR of LN metastasis by two-year completion of 1st line Ate+Beva therapy in advanced HCC patient. We need to observe further real-life data in south Korea regarding further treatment strategy after two-year completion of Ate+Beva therapy.
NOTES
-
ACKNOWLEDGEMENTS
None.
-
FUND
None.
-
ETHICS STATEMENT
The present case report was approved by the Institutional Review Board (IRB) of the Dongnam Institute of Radiological & Medical Sciences, Busan, Korea (IRB number: D2512-001-002).
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception: SYH, WJ. Manuscript preparation: SYH. Critical revision: SYH, WJ. All authors reviewed the paper and approved the final version.
Figure 1.Liver CT scan. Huge portocaval lymph node (6 cm sized) metastasis was observed. CT, computed tomography.
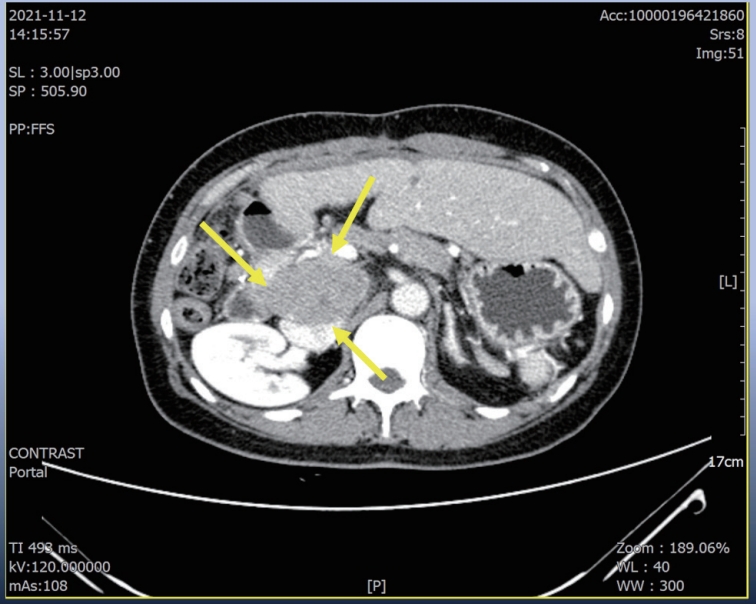
Figure 2.Liver CT scan (A–C) and chest CT scan (D) after seven cycles of 1st line Ate+Beva therapy. Almost peritoneal metastasis were shrinked (A). However prerenal (B) and peripancreatic (C) lymph node metastasis were increased and new onset lung metastasis was detected (D). CT, computed tomography; Ate+Beva, atezolizumab plus bevacizumab.
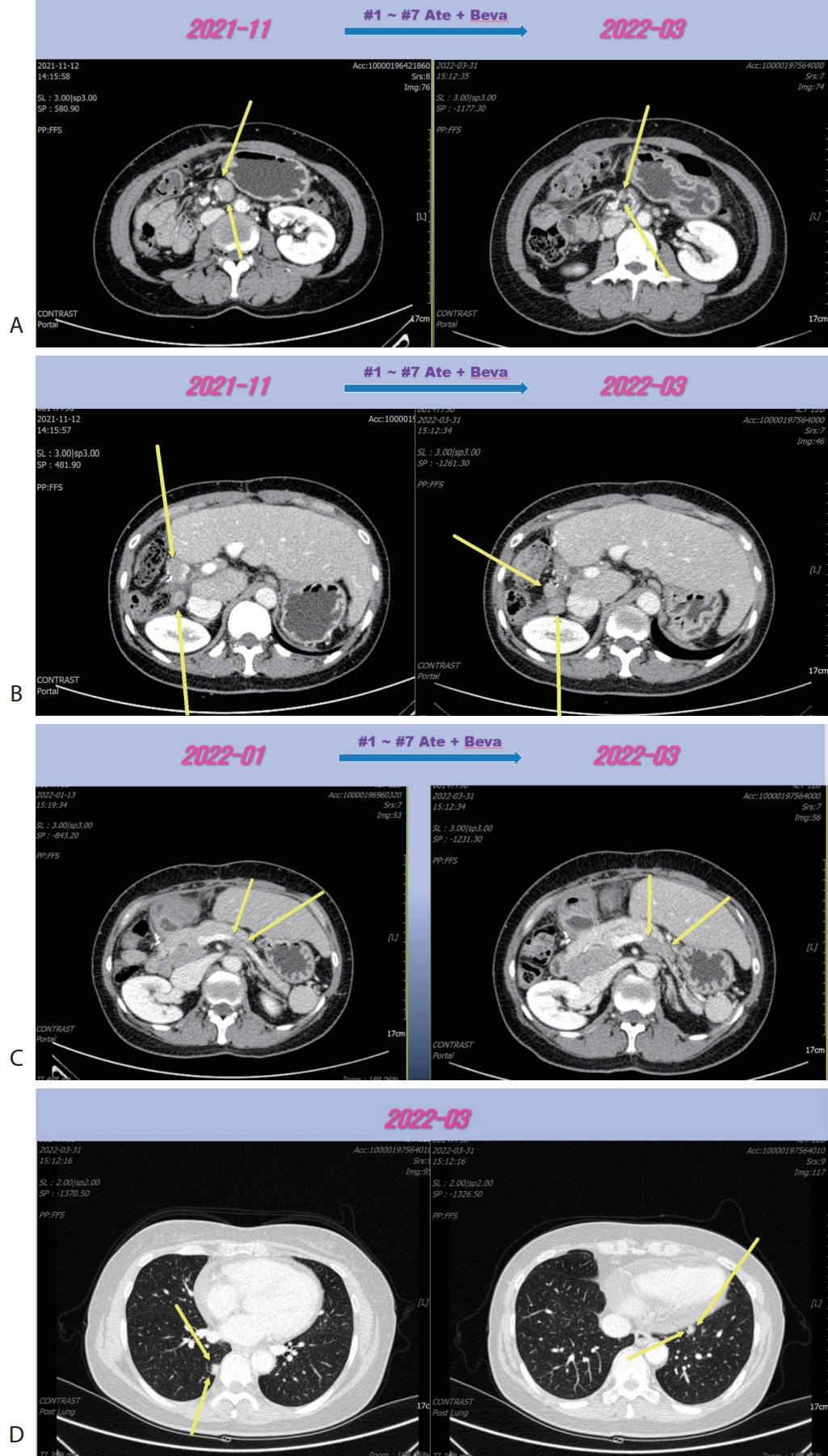
Figure 3.Liver CT scan (A–C) and chest CT scan (D) after combination therapy of RT and fifteen cycles of 1st line Ate+Beva. Increasing peritoneal and lymph node metastasis were shrinked (A, B). Especially although RT was irradiated to only the prerenal lymph node metastasis (A), out-field site of RT such as peripancreatic (B), largest portocaval lymph node metastasis (C) and new onset lung metastasis (D) were markedly improved. CT, computed tomography; RT, radiotherapy; Ate+Beva, atezolizumab plus bevacizumab.
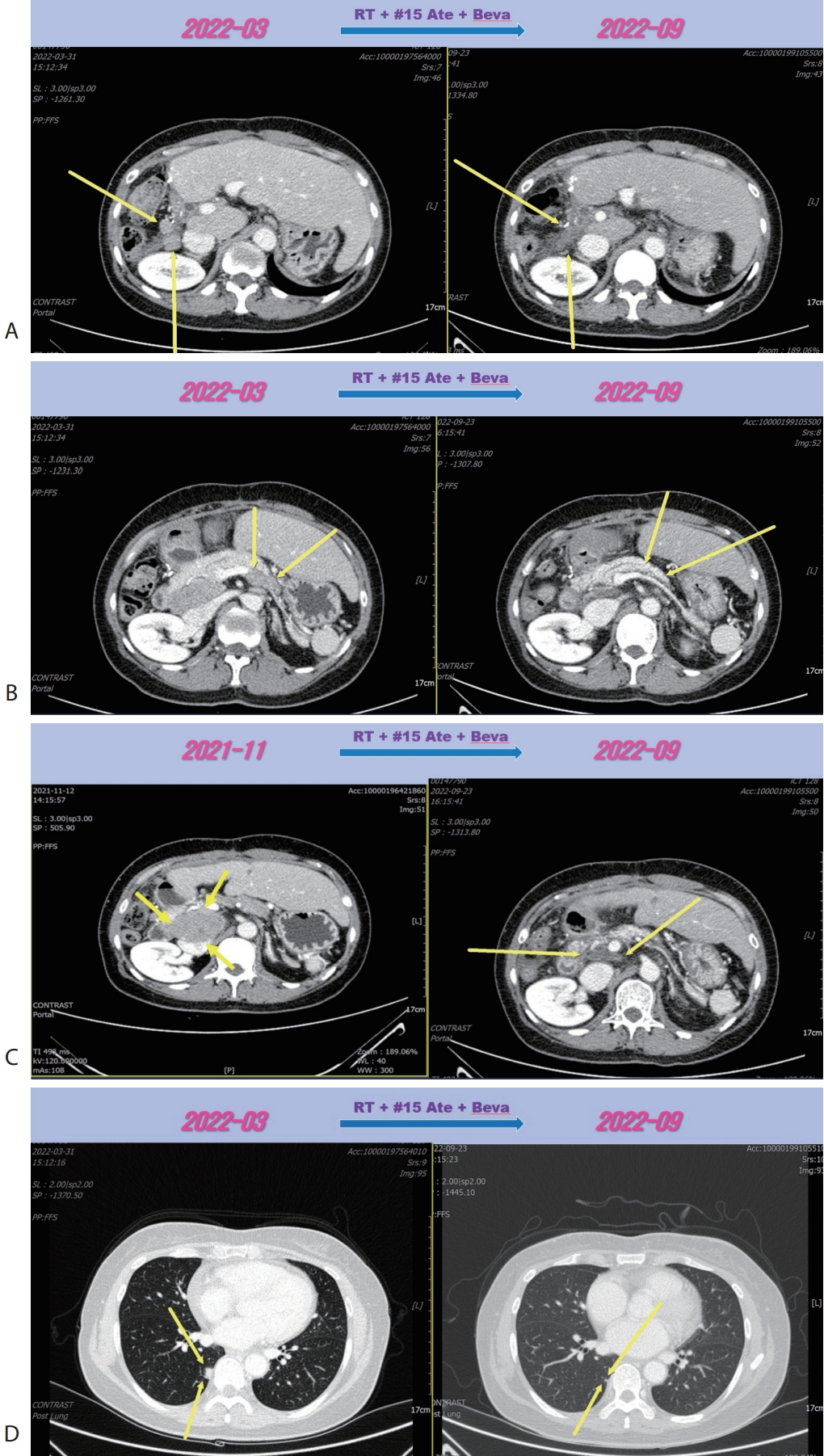
Figure 4.Liver CT scan (A, B) after 2nd line sorafenib therapy. New developed pelvic (A) and inguinal (B) lymph node metastasis were progressed after sorafenib therapy. CT, computed tomography.
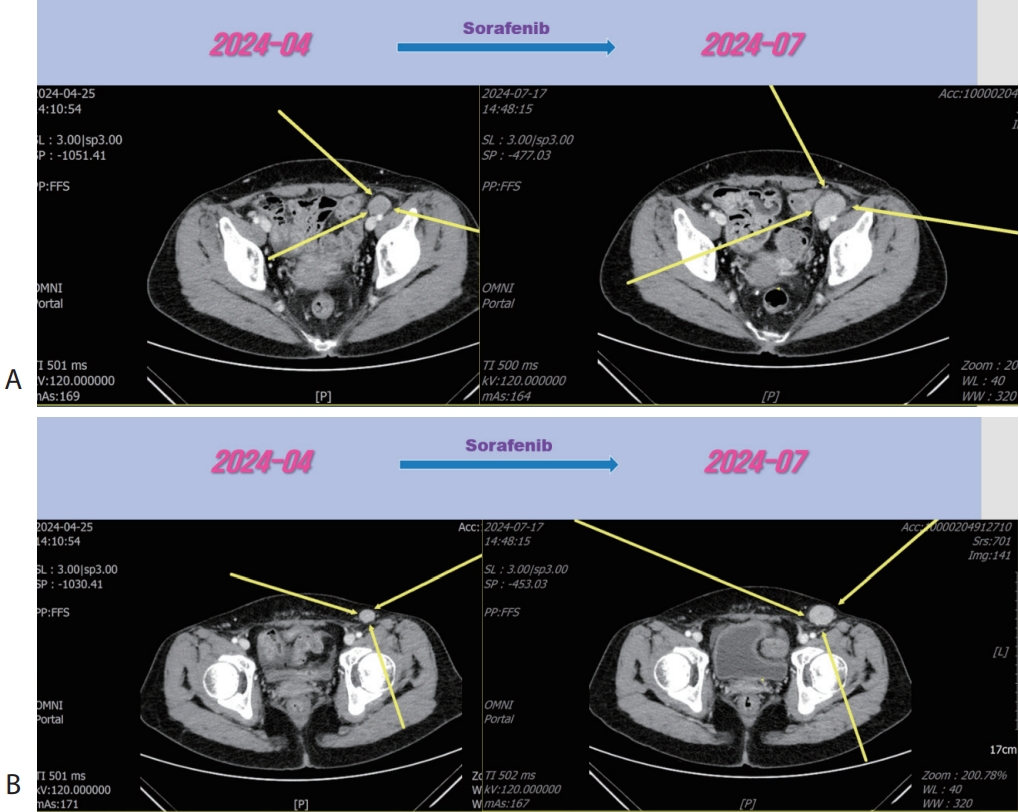
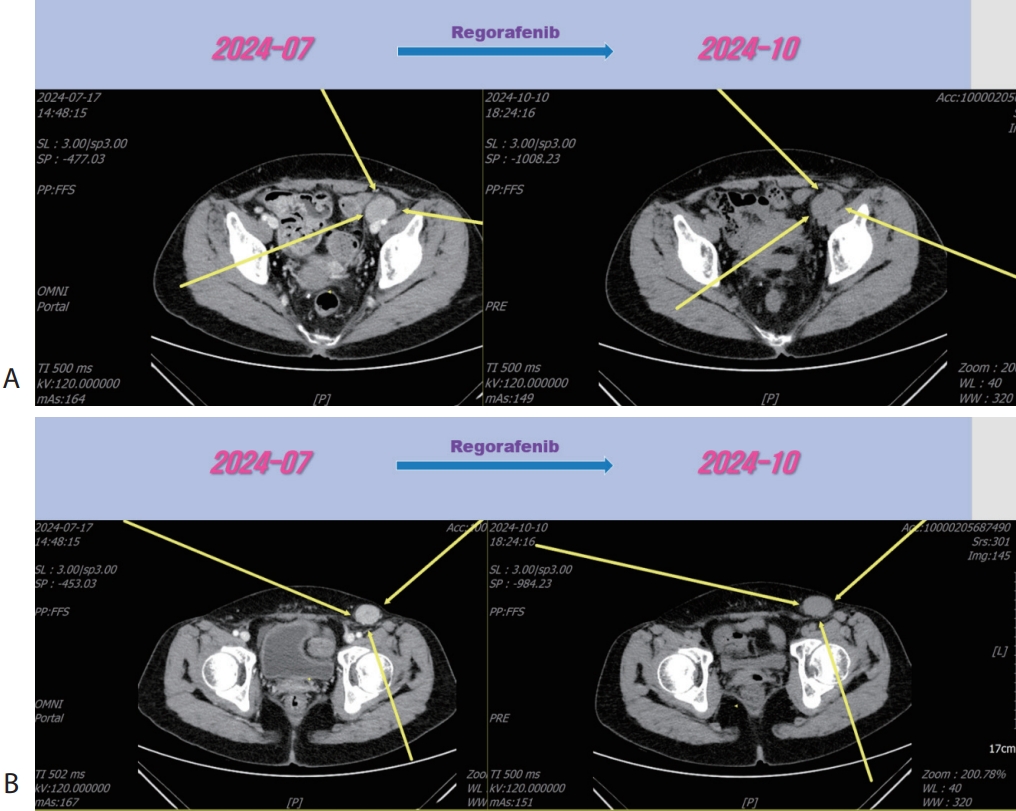
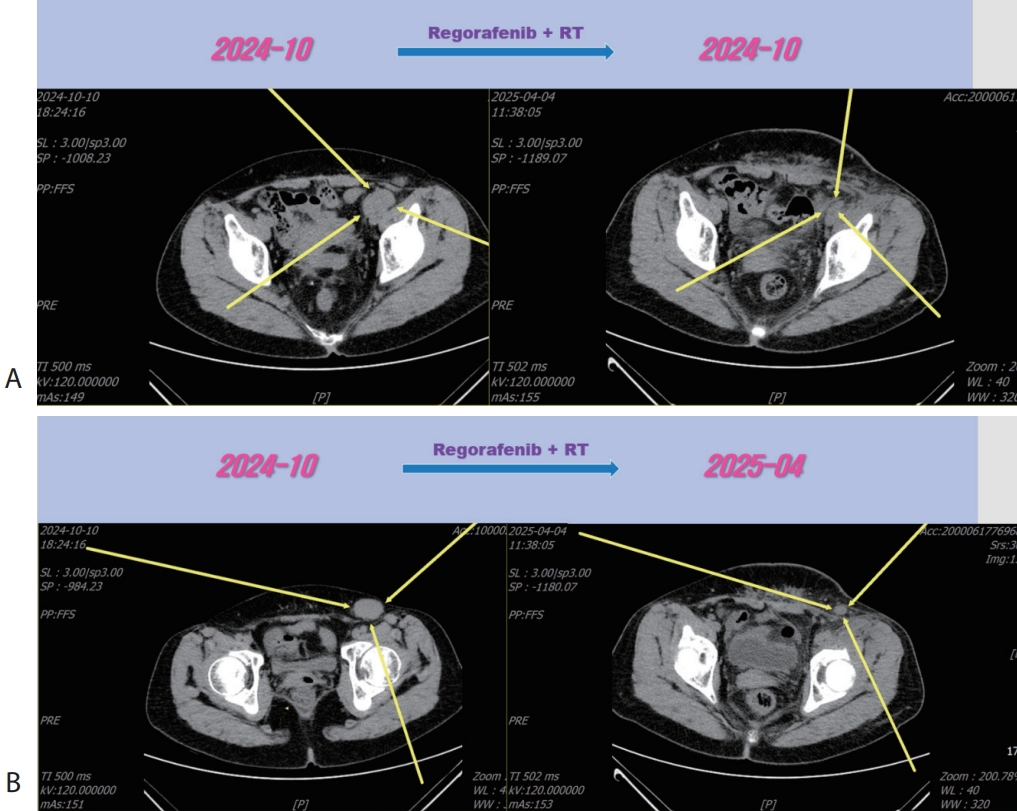
Figure 5.Liver CT scan (A, B) after 3rd line regorafenib therapy. Pelvic (A) and inguinal (B) lymph node metastasis were slightly increased after regorafenib therapy. CT, computed tomography.
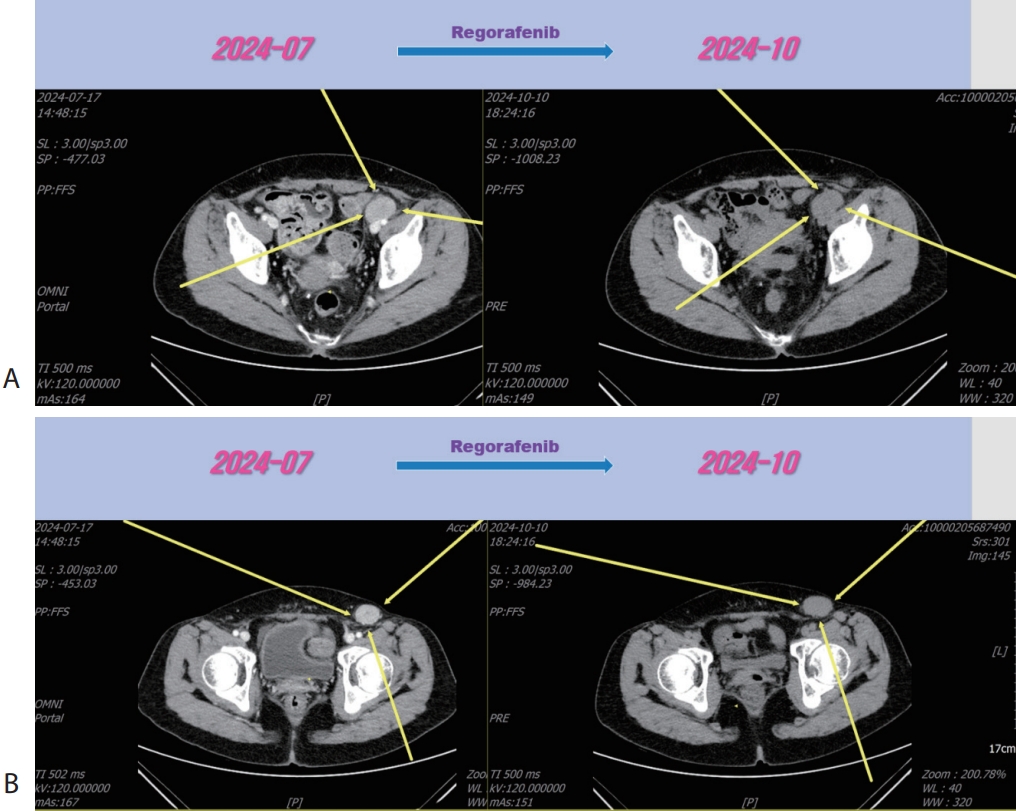
Figure 6.Liver CT scan (A, B) after combination therapy of RT and 3rd line regorafenib. Slightly increasing pelvic (A) and inguinal (B) lymph node metastasis were marked shrinked after combination therapy. CT, computed tomography.
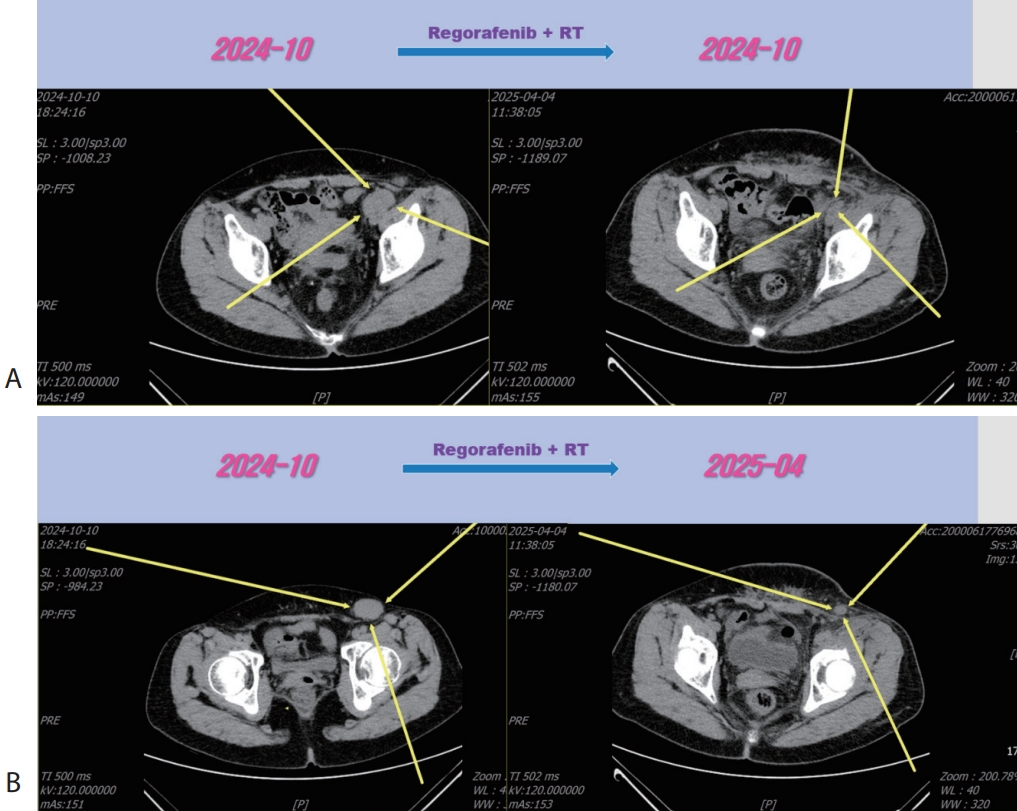
Figure 7.Summary of treatment timeline of patient. Ate+Beva, atezolizumab plus bevacizumab; RT, radiotherapy; Gy, gray; Fr, fraction; CR, complete response.

REFERENCES
- 1. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894-1905.
- 2. Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019;380:1450-1462.
- 3. Han JW, Sohn W, Choi GH, et al. Evolving trends in treatment patterns for hepatocellular carcinoma in Korea from 2008 to 2022: a nationwide population-based study. J Liver Cancer 2024;24:274-285.
- 4. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022;76:681-693.
- 5. Yoo C, Kim JH, Ryu MH, et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: a multinational multicenter retrospective study. Liver Cancer 2021;10:107-114.
- 6. Persano M, Rimini M, Tada T, et al. Sequential therapies after atezolizumab plus bevacizumab or lenvatinib first-line treatments in hepatocellular carcinoma patients. Eur J Cancer 2023;189:112933.
- 7. Lee CK, Yoo C, Hong JY, et al. Real-world study of systemic treatment after first-line atezolizumab plus bevacizumab for hepatocellular carcinoma in Asia-Pacific Countries. Liver Cancer 2024;14:127-141.
- 8. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66.
- 9. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54-63.
- 10. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282-296.
- 11. Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with Sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol 2020;6:e204564.
- 12. Prelaj A, Pircher CC, Massa G, et al. Beyond first-line immunotherapy: potential therapeutic strategies based on different pattern progressions–oligo and systemic progression. Cancers 2021;13:1300.
- 13. Sindhu KK, Nehlsen AD, Lehrer EJ, et al. Oligoprogression of solid tumors on immune checkpoint inhibitors: the impact of local ablative radiation therapy. Biomedicines 2022;10:2481.
- 14. Kim DH. Combination of interventional oncology local therapies and immunotherapy for the treatment of hepatocellular carcinoma. J Liver Cancer 2022;22:93-102.
Citations
Citations to this article as recorded by